60601 impact test|what is iec 60601 : importing IEC 60601-2- x standards. This booklet describes the electrical safety requirements for compliance with IEC 60601-1. Although a type of test standard, most of these tests are used . Resultado da Casino Sun City Ica, Ica, Peru. 2,754 likes · 5 talking about this · 502 were here. ¡Bienvenido al Fan Page Oficial de Casino Sun City ! Entérate de.
{plog:ftitle_list}
Resultado da 4 de abr. de 2023 · A provável escalação do Leeds para a partida é: Mesiler; Ayling, Koch, Struijk, Firpo; Harrison, Kristensen e Marc Roca; Sinisterra, Aaronson, .
IEC 60601 impact testing requirements are nerve-wracking tests. Blog includes 6 tips plus video to help you understand the methods and avoid .IEC 60601-2- x standards. This booklet describes the electrical safety requirements for compliance with IEC 60601-1. Although a type of test standard, most of these tests are used .
I have a question about the ball impact test requirements in IEC 60601-1. For body worn devices the ball impact test applies. How about if the device is tiny, like a hearing .Annex D provides an overview of the IEC 60101-1-X and IEC 60601-2-X standards. This booklet describes the electrical safety requirements for compliances with IEC 60601-1. Although a type . Nigel Syrotuck, Mechanical Engineering Lead at StarFish Medical, demonstrates how to conduct an IEC-60601-1 Medical Device Push Test as part of his companion blog on . Impact testing. When testing impact, you should be aware of the appropriate transport speed for your medical equipment cart. You must move the cart as fast as you usually would, at .8 m per second, for an accurate test. .
Download our guide and understand the commonly used definitions withing IEC 60601, testing requirements, and the importance of regular device testing in order to keep people safe. IEC 60601-1 is used both by technicians out in the field, manufacturers hoping to receive certification for a medical electrical device (ME device), and the test & certification houses who test ME devices for . IEC 60601-1 is a technical standard that medical electrical equipment and devices must meet to be considered safe and effective before they go to market. It’s required for FDA approval, and devices must meet the .
In 60601-1; 15.3.3, it is stated that the impact test is not applicable for HAND-HELD ME EQUIPMENT. Does anyone know the reason behind this test not being applicable for hand-held? "Except for HAND-HELD ME EQUIPMENT and ME EQUIPMENT parts that are HAND-HELD, ENCLOSURES and other external insulating parts, the deterioration of which . That will impact the specific standards that are applicable. Each region has different versions with slightly varied requirements: International: IEC 60601-1:2005; EU: . By testing at every step, you will be confident of the . But we’ll do our best in the pages that follow. About the IEC Amendments Project. The Amendments Project under SC62A covers the general standard (IEC 60601-1) and most of the collateral standards (IEC 60601-1-XX, . Hi, We are designing a portable medical device with a glass face which has two separate subareas: a) the display area and b) the touch controller/buttons area. According to the IEC 60601-1 standard, the display area should be excluded from the 15.3.3 Impact test. "The test is not applied to flat panel displays, to the platen glass of ME EQUIPMENT (for example film .
We offer end-to-end solutions from product development, to review of the Risk Management File and supporting documentation, to comprehensive testing to the 60601/80601 series of standards, including an array of Collateral (IEC 60601-1-X) and Particular (IEC 60601-2-X) Standards.15.3.3 Impact Test 15.3.4 Drop Test 15.3.6 Moulding Stress Relief 15.5.1.2 Transformer short-circuit test . IEC 60601-1 Clause Requirement + Test Result - Remark Verdict TRF No. IEC60601_1J_PS INSULATION DIAGRAM Class II / Double .
IEC 60601-2- x standards. This booklet describes the electrical safety requirements for compliance with IEC 60601-1. Although a type of test standard, most of these tests are used to test medical devices, both regularly and following service or repair. Introduction to IEC 60601 Local Adaptation In many cases the IEC 60601 standard has Follow these tips to adhere to proper 60601 testing for your custom medical cart. . Impact testing. When testing impact, you should be aware of the appropriate transport speed for your medical equipment cart. You must move the cart as fast as you usually would, at .8 m per second, for an accurate test. . The ball-impact test is an addition to the requirements in IEC 60601-1, and the drop test is a modification of the test requirements called for in IEC 60601-1. The ball-impact test is conducted on the top, sides, and front surfaces of the device under test .Since the publication of the IEC versions of 60601-1 in late 2020, our main testing laboratories across the U.S., Europe and Asia have achieved Certified Body Testing Laboratory (CBTL) status. Dedicated to healthcare industry innovation, UL Solutions leverages decades of technical, regulatory and clinical expertise to help you manage regulatory .
How to define IEC 60601 test plans and protocols for medical devices. Published on: February 17, 2021; All articles, Electrical medical device safety IEC 60601-1; This article will go over the process of writing test plans and protocols for medical devices in line with the IEC 60601 standard. These documents are essential in order to .IEC 60601 is a series of technical standards that ensure the safety of medical electrical equipment. IEC 60601-1 (Edition 3.1) serves to ensure that no single electrical, mechanical or functional failure shall pose an unacceptable risk to patients and/or operators. With 60601 series of standards, the testing required is “type testing” as opposed to the process-based standards such as IEC 62366 (Usability Engineering Process) or IEC 62304 (Life-cycle Software process). . Complex EP can impact testing time as some tests will need to be repeated to confirm it as a criteria for compliance. 13. Pre-test .
hydraulic testing near me
IEC 60601 1 11 the test standard assigns categories to devices and these categories are based on whether the equipment is designed to be stationary or transported during normal use. . Complete, Filled Transport Packages and Unit Loads, Impact Test with Rotary Drop; ISO 2247 Packaging, Complete, Filled Transport Packages and Unit Loads .
what is iec 60601
Impact Test Balls The Impact Test Ball is a specially machined ball to test impact resistance according to IEC, CSA and UL Standards. It is made of chrome-plated-steel for durability, and is fitted . IEC 60601-1:2005, IEC 61032:1998 ITB15 50mm diameter ball with eyebolt 500 grams IEC 60529, 61032 ITB20 50 mm diameter ball with handle NR ASTM .
The need for validation testing is driven by 21 CFR Part 820.30(g) which states “Design validation shall ensure that devices conform to defined user needs and intended users and shall include testing of production units under actual or simulated use conditions.” Furthermore, the FDA has had an increased enforcement of Human Factors since .The Impact Test Ball is a specially machined ball to test impact resistance according to IEC, CSA and UL Standards. It is made of chrome-plated steel for durability, and is fitted with an eyelet to facilitate testing for pendulum . IEC 60601-1:2005 15.3.3 IEC 61032:1998 Figure 5, Test Probe 1 IEC 60950 Section 4.2.5 IEC 61010-1 Clause 8.2.1 . Bodies, Testing Laboratories, and. Food and Drug Administration Staff. Document issued on . September 25, 2020. The draft of this document was issued on . September 23, 2019.
hydraulic testing tools
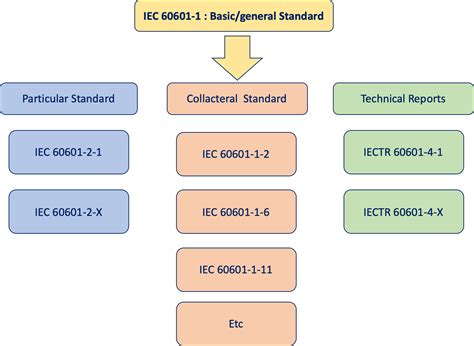
Drop Ball impact Test; Ear Probe for Audiometry; Earthing Enhancing Testing According to IEC 62561-7:2018; Electric Cable Testing for Photovoltaic Systems; Electrical Motor Testing; . IEC 60601 Testing; IEC 60601 Testing. Elevate your electro-medical devices standards;IEC 60601 is a series of technical standards for the safety and essential performance of medical electrical equipment, published by the International Electrotechnical Commission. First published in 1977 and regularly updated and restructured, as of 2011 it consists of a general standard, about 10 collateral standards, and about 80 particular .The UIH20 meets the testing requirements of industry standards such as IEC 60068-2-75:2014, as well as IEC 60065, 60335, 60598, 60601 and 61010. The hammer simulates mechanical impact to electronic products and electrical appliances. Certification testing to 60601-1 must typically be completed formally by a third party laboratory before a device can be approved for sale. However, formal third party testing can take weeks or even months, and if there is a design issue or failure that needs to be resolved, it can take even longer. .
From prototype evaluations and pre-compliance testing to full-compliance testing and certification, our state-of-the-art 3-, 5-, and 10-meter EMC chambers are equipped to evaluate your medical device to requirements within the EMC Directive, including IEC 60601-1-2 and IEC 60601-2-x particular standards. Conforming to the IEC 60601-1 standard. The reason the standard is important to manufacturers is that their devices need to conform to it in order for the devices to meet the requirements of Medical Devices Regulations such as MDR in the EU. In order for them to prove that this is the case, the device shall be subject to compliance assessment which is normally .IEC 60601-2-52 Impact Test Device, Movement Parts Impact Test Device. Product Description . IEC 60601-2-52 Rough Handling And Threshold Impact Test Device For Movement Parts Test Product Information: 1. Standard: conform to IEC60601-1:2012, YY9706.252-2021 clause 201.9.4.2.4.3 and 201.15.3.5, IEC60601-2-52:2015. 2. Samples and restrictions .withstand impact testing per IEC 60601-1 clause 15.3.3 . while powered on. System meets basic safety and essential performance requirements following impact test. The enclosure parts shall have no rough surfaces, sharp edges or corners that could result in injury or damage. Met .
On Sept. 1, 2020, Amendment 1 of IEC 60601-1-2:2014 was published and can be considered already applicable. The main changes impact the immunity test to be performed to medical equipment and medical systems. In fact, according to IEC 61000-4-39, Electromagnetic compatibility, an additional immunity test needs to be performed.
six 60601 impact testing
webOficinas Mecânicas de Motos em Bom Despacho - Minas Gerais mais perto de você. Encontre aqui mais de 6 empresas cadastradas. Veja o telefone, endereço, localização e saiba como chegar. Se você procura serviços de revisão, manutenção preventiva e reparos em sua moto, como: Balanceamento de Rodas de motos em Bom Despacho;
60601 impact test|what is iec 60601